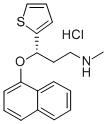
Duloxetine CAS 136434-34-9

Name:Duloxetine hydrochloride
CAS:136434-34-9
MF:C18H20ClNOS
MW:333.88
EINECS:603-962-5
Duloxetine, sold under the brand name Cymbalta among others,[1] is a medication used to treat major depressive disorder, generalized anxiety disorder, fibromyalgia, and neuropathic pain.[5] It is taken by mouth.[5]
Common side effects include dry mouth, nausea, feeling tired, dizziness, agitation, sexual problems, and increased sweating.[5] Severe side effects include an increased risk of suicide, serotonin syndrome, mania, and liver problems.[5] Antidepressant withdrawal syndrome may occur if stopped.[5] There are concerns that use during the later part of pregnancy can harm the baby.[5] It is a serotonin–norepinephrine reuptake inhibitor.[6] How it works is not entirely clear.[5]
Duloxetine was approved for medical use in the United States and in the European Union in 2004.[5][3][4] It is available as a generic medication.[6] In 2018, it was the 36th most commonly prescribed medication in the United States, with more than 21 million prescriptions.
CAS:136434-34-9
MF:C18H20ClNOS
MW:333.88
EINECS:603-962-5

Duloxetine, sold under the brand name Cymbalta among others,[1] is a medication used to treat major depressive disorder, generalized anxiety disorder, fibromyalgia, and neuropathic pain.[5] It is taken by mouth.[5]
Common side effects include dry mouth, nausea, feeling tired, dizziness, agitation, sexual problems, and increased sweating.[5] Severe side effects include an increased risk of suicide, serotonin syndrome, mania, and liver problems.[5] Antidepressant withdrawal syndrome may occur if stopped.[5] There are concerns that use during the later part of pregnancy can harm the baby.[5] It is a serotonin–norepinephrine reuptake inhibitor.[6] How it works is not entirely clear.[5]
Duloxetine was approved for medical use in the United States and in the European Union in 2004.[5][3][4] It is available as a generic medication.[6] In 2018, it was the 36th most commonly prescribed medication in the United States, with more than 21 million prescriptions.
【 Go Back 】





